Radioactivity Explained
1. Atomic structure
Putting you in the picture
1.1 Atoms and elements
1.2 Structure and scale of the atom
1.3 Atomic number, mass number and isotopes
1.1 Atoms and elements
Short video summary (2:59)
-
The ideas of atoms and elements is very ancient and occurred in many cultures
Elements are the simplest substances
Atoms are the smallest parts
John Dalton combined the idea of elements and atoms in the early 1800’s
Radioactivity showed Dalton’s assumptions that atoms were permanent, unchanging and indivisible were wrong
-
Are all trees made from the same type of stuff?
If so, how many types of stuff?
And what about rocks and rivers - do they share anything in common with trees?
Humans like to find patterns in nature, and this has led different cultures to think of everything being made from a few fundamental substances, which we call elements.
There was a surprising amount of overlap for peoples who had never had contact with each other.
The ancient Greeks and Native Americans believed everything was made up from combining earth, air, fire and water.
Hindus added an airy substance called ether to these four.
And the Chinese didn't include air but did have metal and wood [earth, fire, water, metal and wood].
A related question is do things change or do they stay the same?
Think of a river - it changes from moment to moment, but it's still the same river.
One way to reconcile this problem is to think of little particles that don't change over time but recombine in different ways - the particles stay the same but what they make up changes.
Lots of cultures have developed some variation of this model.
The ancient Indian philosopher Kanada believed that different atoms have different properties that give things colour, taste or smell.
The ancient Greek philosopher Democritus believed that there were an infinite number of types of atom.
Atomic theory has gone in and out of fashion in different parts of the world at different times.
In the early 1800's John Dalton developed his chemical atomic theory which combined the ideas of elements and atoms.
Essentially it said that everything was made from atoms with empty space in between them.
All atoms were the same apart from their mass.
Each element had atoms of a specific mass - so all oxygen atoms had one mass and all iron atoms another.
Dalton only knew of about forty elements - now we know that there are 94 that occur naturally on Earth.
Every atom was eternal (it had been around forever and always would be), immutable (an atom of one element couldn't turn into an atom of another element) and indivisible (you couldn't split atoms up into smaller pieces).
We're going to see that over the following hundred years each one of those assumptions would be proved wrong.
The study of radioactivity would show that you could break atoms into pieces to make new atoms, and cause an atom of one element to change into an atom of a different element.
-
The ideas of atoms and elements in ancient cultures were the result of thinking very hard, rather than doing experiments.
Dalton’s atomic theory was a result of trying to explain why chemicals seemed to always combine in the same proportions by mass.
It’s this ability to use numbers in experimental predictions that puts Dalton firmly in the science, rather than the philosophy, camp.
Key animations from the video for you to use in front of a class
Slideshow: Atoms and elements
1.2 Structure and scale of the atom
Short video summary (2:14)
-
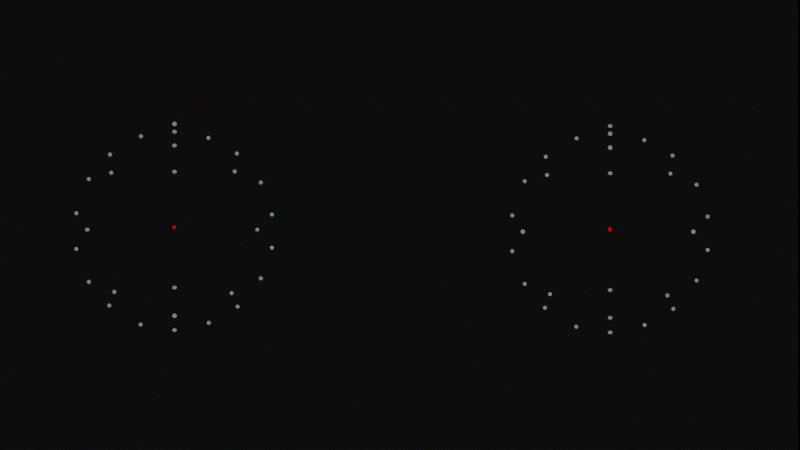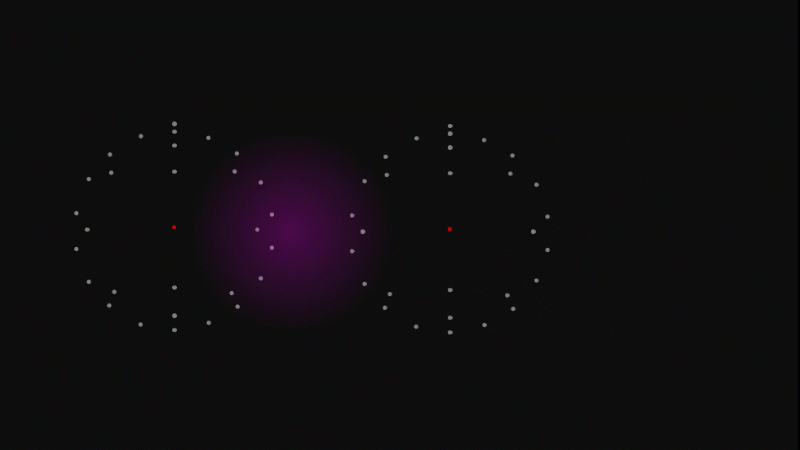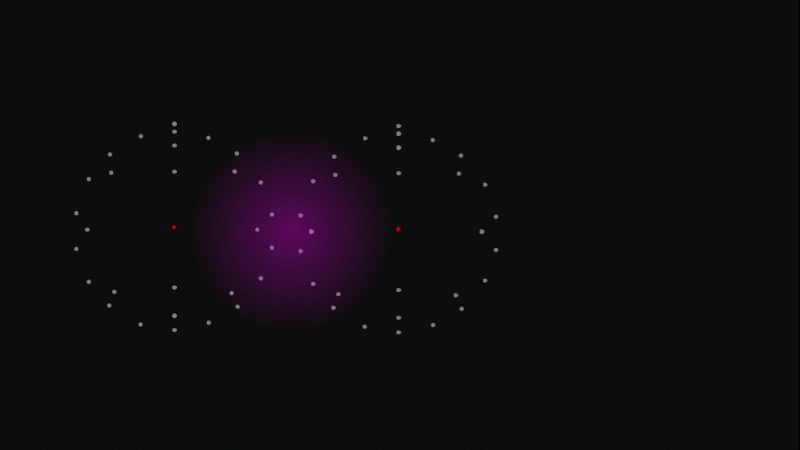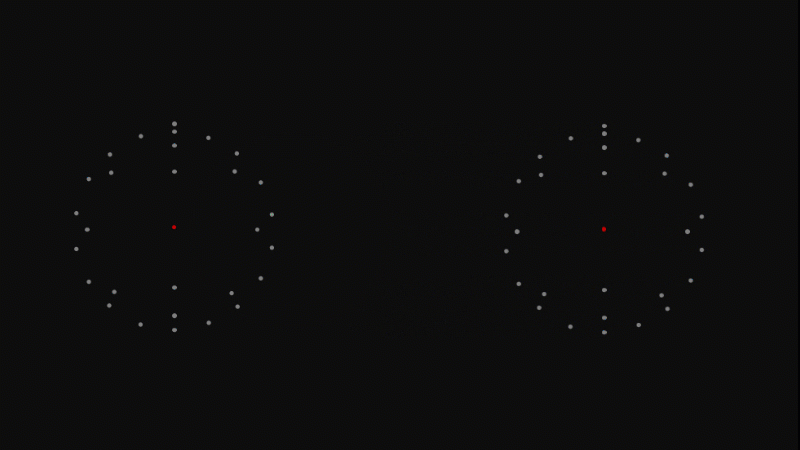
Atoms are made of a positive nucleus surrounded by negative electrons
The nucleus is tiny compare to the rest of the atom
The nucleus is made from protons and neutrons
Most of an atom is empty space, and there is empty space between atoms
-
Let's look at our modern picture of an atom first and then in Lesson 2 we'll look at the history of how we arrived at this picture.
We now know that atoms consist of two regions.
In the centre is the incredibly tiny and dense nucleus - this is where almost all of the mass of an atom is to be found.
Around the nucleus are whizzing electrons.
Sometimes we imagine these as like planets orbiting a star.
Sometimes we imagine them as like clouds.
The nucleus has an overall positive electrical charge.
The electrons have a negative electrical charge.
In a neutral atom the positive charge on the nucleus is cancelled out by the negative charge on the electrons, and so the atom has no overall electric charge.
An atom is almost entirely empty space, and what's between atoms is also just empty space.
So you me, table, chairs, mountains and tangerines and every other kind of stuff is mostly empty space.
It doesn't feel empty because the electrons repel so strongly if you try and push atoms on the surface of one object too close to atoms on the surface of another.
The nucleus is tiny - if the whole atom was the size of a sports stadium then the nucleus would be about the size of a grape.
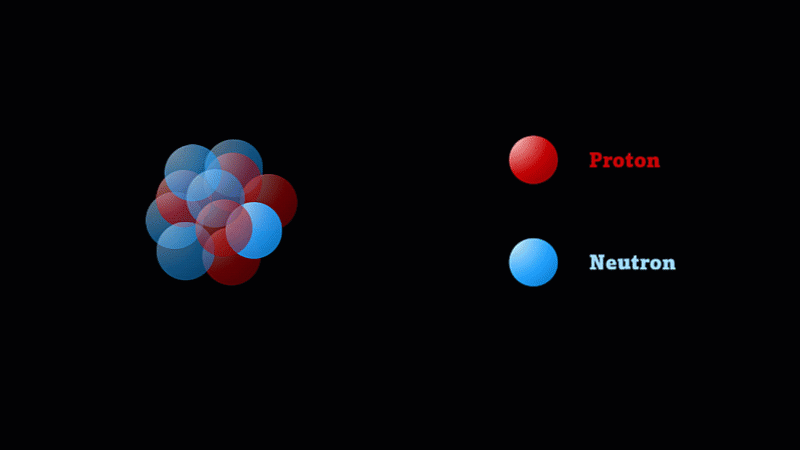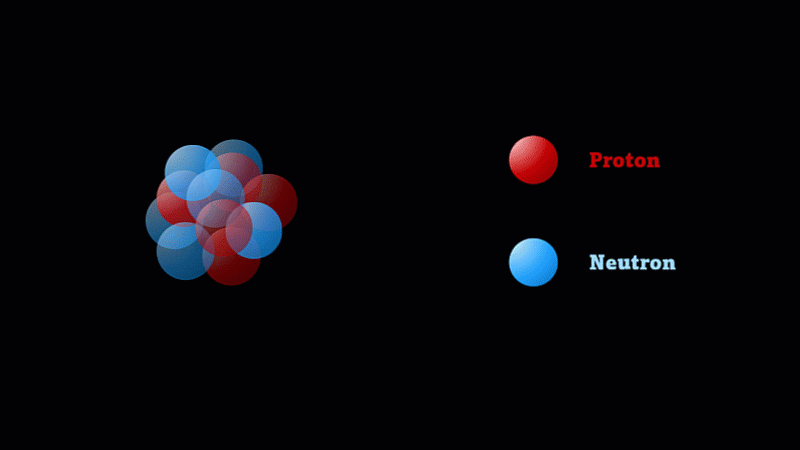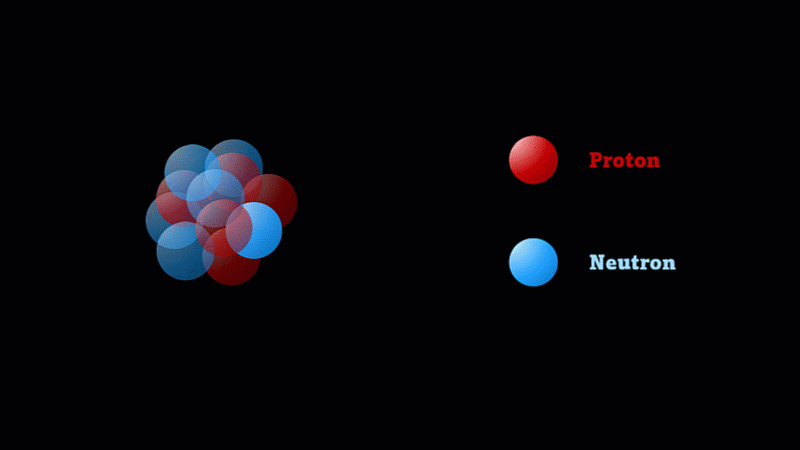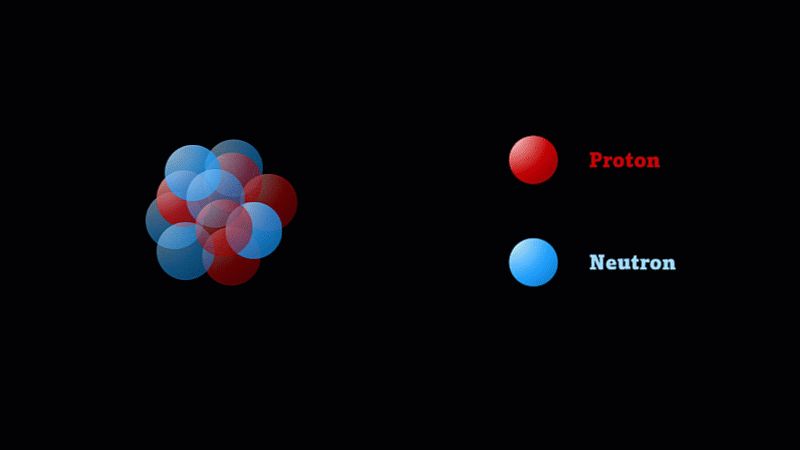
But the nucleus itself is made of smaller parts called protons and neutrons.
Protons have a positive charge and neutrons have no electric charge.
When we study radioactivity, it's the nucleus we're interested in, not the electrons.
When we talk about 'a nucleus' we just mean the nucleus at the centre of an atom - the rest of the atom is still there - the empty space and the electrons - but we just ignore it.
-
This is a fairly straight description of an atom as we use it commonly today.
By introducing it first, we give students a spoiler about how the history of the study of the atom will end.
It also starts giving students a useful vocabulary, which we’ll use all the way through the course.
It’s important to spend some time emphasising this idea that when we talk about the nucleus, the rest of the atom is still there - we just ignore it.
This is only untrue when we talk about extreme or non-terrestrial radiation like the centre of stars, and cosmic rays.
Key animations from the video for you to use in front of a class
Atoms are mostly empty space
Atomic nucleus and electrons
Positive nucleus and negative electrons
The nucleus is like a grape in a stadium
Electrons repel so things feel solid
Protons and neutrons make up a nucleus
1.3 Atomic number, mass number and isotopes
Short video summary (3:54
-
An element is defined by the number of protons in the nucleus of one of its atoms
This is called its atomic number (or proton number, or nuclear charge)
The total number of protons and neutrons is called the mass number (or nucleon number)
Different atoms of the same element can have different numbers of neutrons
We use the word ‘isotope’ to describe which flavour of an atom we’re talking about
Isotopes are defined by their mass number, for example carbon-12
-
Since we're going to be talking about the nucleus of atoms, it's useful to have some words we can use to describe the nucleus.
The nucleus of a boron atom has 5 protons and 5 neutrons.
It's the 5 protons that makes it boron.
Boron is element number 5 in the periodic table.
When we're talking about nucleuses we put a little number at the bottom left of our element symbol to remind us how many protons that nucleus has.
In chemistry we put a little number at the bottom right to indicate how many atoms there are in a molecule - they don't mean the same thing.
Fortunately they're rarely used in the same place at the same time.
We call this little number the atomic number because it tells you the element of which atom it is in the periodic table.
It's equal to the nuclear charge because each proton has an electric charge of +1, if we say electrons have an electric charge of -1.
Carbon has an atomic number of 6. The 6 protons in every carbon nucleus are what makes it carbon - and it's element number 6 in the periodic table.
Nitrogen has an atomic number of 7. The 7 protons in every nitrogen nucleus are what makes it nitrogen - and it's element number 7 in the periodic table.
As well as its 5 protons, a boron nucleus also has 5 neutrons.
Protons and neutrons have roughly the same mass, so we say the mass number of this boron nucleus is 10.
Using these two numbers - the atomic number and the mass number - we can define any nucleus.
The atomic number tells us the number of protons, and therefore what element it is, and the mass number tells us the total number of protons and neutrons.
For short we'd call this nucleus boron-10.
Every atom has a fixed number of protons that defines what element it is, and roughly the same number of neutrons.
Different atoms of the same can have different numbers of protons.
For example, imagine we have two carbon nucleuses. Each will have 6 protons.
One has 6 neutrons giving it a mass number of 12.
The other has 8 neutrons giving it a mass number of 14.
If you wanted to ask which variety of carbon nucleus we're talking about you'd ask ‘Which isotope of carbon are we talking about?'
Isotope means 'same place' in the periodic table.
They're both carbon, but they have different masses, so they're different isotopes.
Carbon-12 is the most common isotope of carbon, but in the atmosphere about one carbon atom in a trillion is the isotope carbon-14.
Carbon-14 and nitrogen-14 are both isotopes - they're just not istopes of the same element.
Carbon-14 is an isotope of carbon. Nitrogen-14 is an isotope of nitrogen.
It's the 6 protons that makes carbon-14 carbon.
And it's the 7 protons that makes the nitrogen-14 nitrogen.
-
All atoms are an isotope, in the same way that all animals are a species.
It’s not like there are normal atoms and then there are isotopes. Neither does the idea of an isotope imply radioactivity. There are stable isotopes and there are radioisotopes.
Strictly, the word isotope applies to whole atoms - the equivalent for just the nucleus is a nuclide.
There’s no need to make these fine distinctions with students, but it’s worthwhile understanding the technical language.
Key animations from the video for you to use in front of a class
Slideshow: Mass number and isotopes